TechnologyToday March/April 2021 Column
Click for Printable PDF
To request a free Newsletter subscription email us at:
subscribe@techtoday.us
Click the "Select a Link" Below to Explore
our coverage of our tecnological world.
A Copyright © on the date of publication exists for all articles and other material posted on this website. With proper citation you are welcome to use all the materials in your classroom.
Creating Oxygen and Rocket Fuel on Mars
We need oxygen to breathe and for people to live on Mars they will need oxygen for respiration. They will also need oxygen to fuel their rockets or astronauts will never be able to return to earth. To blast off the red planet, a rocket would need an enormous quantity of rocket fuel (15,000 lbs) and even a greater quantity of oxygen (55,000 lbs). The goal is to eventually be able to manufacture both oxygen and rocket fuel, in the needed quantities, using Martian indigenous materials.
On April 20, 2021 the Perseverance Martian Rover’s MOXIE experimental unit processed a small amount of the Martian atmosphere into breathable oxygen. NASA reports that it took one hour to produce ten minutes of breathable oxygen. Photo 1 shows MOXIE installed in Perseverance.
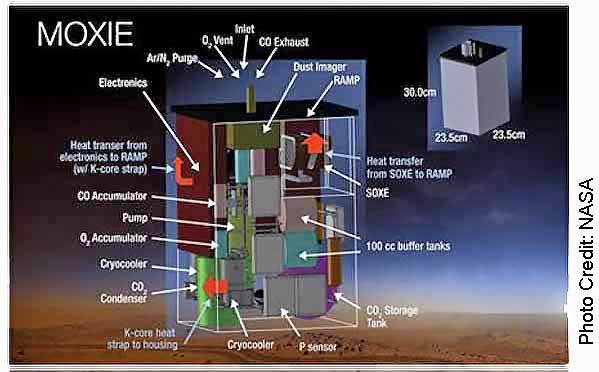
On earth electrolysis fuel cells are used to convert water into hydrogen and oxygen. MOXIE used this same basic process to split carbon dioxide into oxygen and carbon monoxide. Building a machine here on earth that could do this process on Mars was a major accomplishment because it had to be physically small (9.4 X 9.4 X 12.2 inches) light weight (37.7 lb) and run efficiently on very little electricity (300 watts).
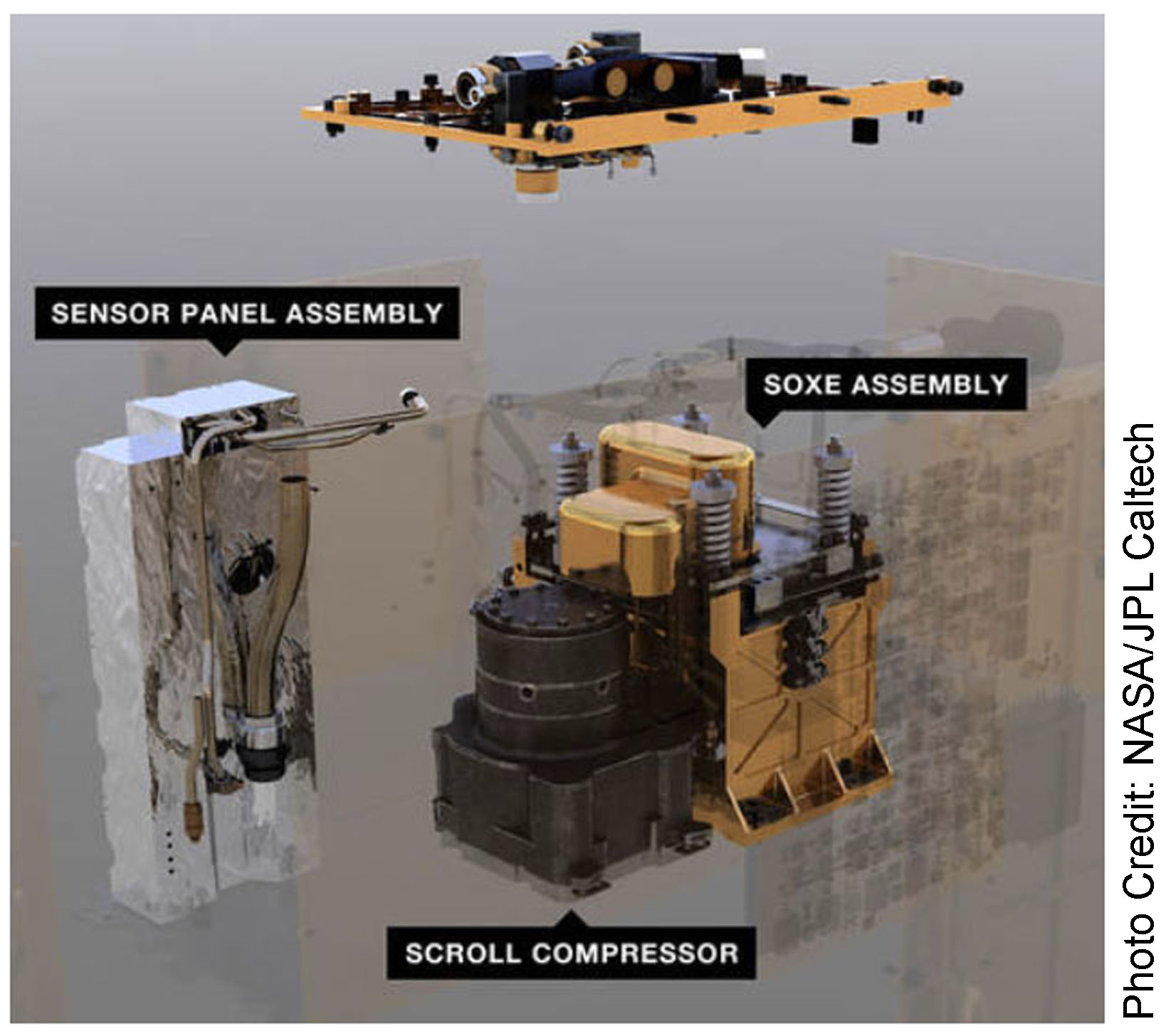
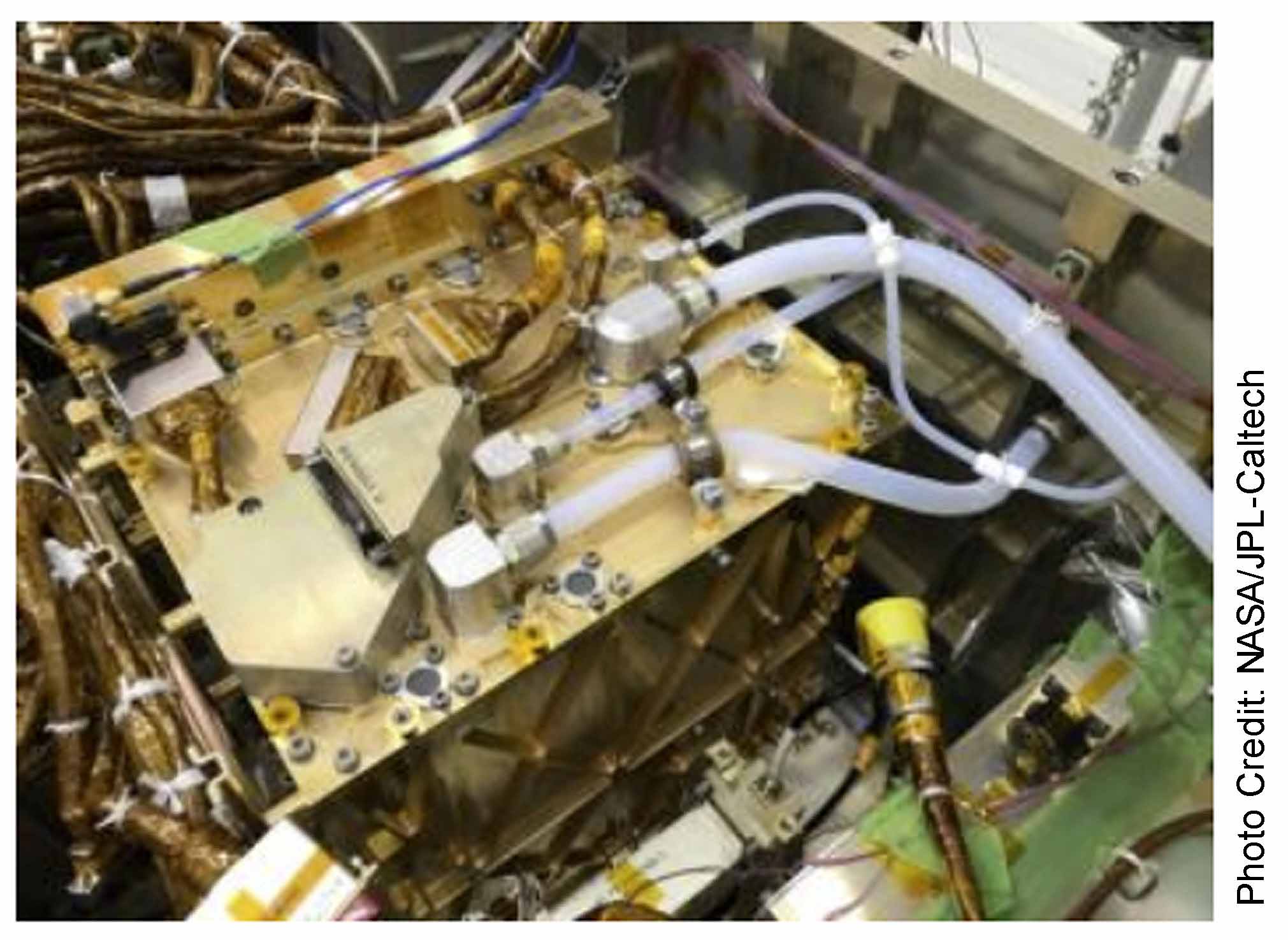
The Martian atmosphere is 95% carbon dioxide. Carbon dioxide (CO2) has one carbon atom and two oxygen atoms. The MOXIE process splits the carbon dioxide molecules (CO2) into O2 and carbon monoxide (CO). To accomplish this task, the Martian atmosphere is first sucked into MOXIE and filtered. The air is then compressed and injected into MOXIE’s Solid OXide Electrolyzer (SOXE) where it is heated to 1,472 degrees Fahrenheit. The processor then electrochemically splits the carbon dioxide into pure oxygen and carbon monoxide. See photo 2.
MOXIE then tested the oxygen to see that it was pure and then released the oxygen and carbon monoxide into the Martian atmosphere. You have to remember that the experiment was a proof of concept and eventually much larger MOXIE units could one day perform the same process, this time sending the oxygen into large storage tanks. The oxygen supply would be built up over time so it will be adequate when astronauts eventually arrive to set up a base on Mars.
NASA describes the process as “the equivalent to running a fuel cell in reverse.” Here on earth hydrogen fuel cells are used to create electricity and on Mars the fuel cell uses electricity to create oxygen.
To blast off from Mars our future settlers now have a proven way to produce the oxygen that they will need. The big question is how do you create the rocket fuel using the natural resources of Mars?
At least two approaches are now on the drawing boards of SpaceX and NASA. SpaceX is researching the possibility of using Mars’ carbon dioxide and Martian frozen water deposits to make rocket fuel. NASA is researching the possibility of using genetically modified CO2 breathing algae that would be sent to Mars. This algae would have all the sunlight and CO2 it needs to propagate on Mars thereby creating the hydrocarbons that could be turned into rocket fuel. For astronauts to visit Mars and return home it is not only a matter of finding a way to create oxygen and rocket fuel because one also needs to be able to supersize experimental solutions so they can create the huge supply needed to blast off the surface of Mars. To learn more watch https://www.youtube.com/watch?v=F1t3J2w4maU&t=39s
Taking it a step further
Online research – Companies on earth are currently creating synthetic fuels using similar technologies to those described in this article. Select one company and describe how their procedure is the same or different than the experimental procedures described in this column to create oxygen or rocket fuel.

To request a free Newsletter subscription email us at: subscribe@techtoday.us